Garnets are ionic materials of general formula XxY2(ZO4)3 that can accomodate a wide range of chemical compositions. They are known for their large diffusion coefficients, which make them promising candidates for technological applications such as lithium-ion batteries. We studied the diffusion of Li+ ions in garnets of composition LixLa3Zr2O12, or LLZ, varying the concentration of lithium from x=3 to x=7.
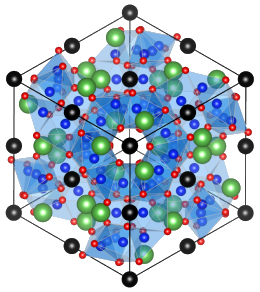
Fig. 1 - Atomic structure of the prototype cubic garnet Li7La3Zr2O12 or "LLZ", viewed along a <111> axis. La3+ ions are in green, Zr4+ in black, Li+ in dark blue and O2- in red. Tetrahedral and octahedral sites for Li+ ions are represented in transparent blue.
Ab initio calculations were employed to determine the ground state for various concentrations of Li+ ions in LLZ garnet. Fig. 2 shows the repartition of Li+ ions among tetrahedral and octahedral sites, as a function of the concentration x of Li+ ions. For the low lithium content x=3, all lithium ions are inside tetrahedral sites, and octahedral sites are all empty. As the concentration of lithium ions is increased, the occupancy of tetrahedral sites decreases, while the occupancy of octahedral sites increases linearly. At very high concentration x=7 all octahedral sites are occupied.
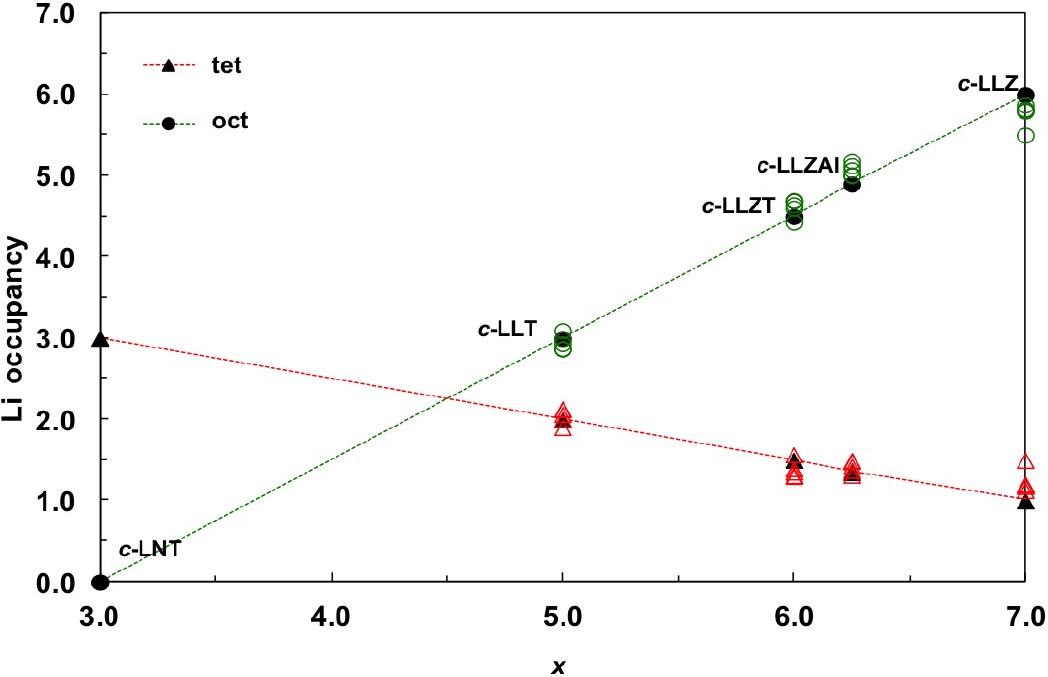
Fig. 2 - Occupancy of tetrahedral and octahedral sites in LLZ garnet as a function of the concentration of Li+ ions.
The diffusion coefficient of the material was computed by molecular dynamics simulations. In turn, this allowed to determine the ionic conductivity as a function of the concentration of Li+ ions, reported in Fig. 3. At low concentration x=3, the conductivity is very low. This is due to the fact that all Li+ ions are in tetrahedral sites, which are disconnected and do not allow Li ions to move easily. As the concentration of Li ions increases, the ionic conductivity also increases.
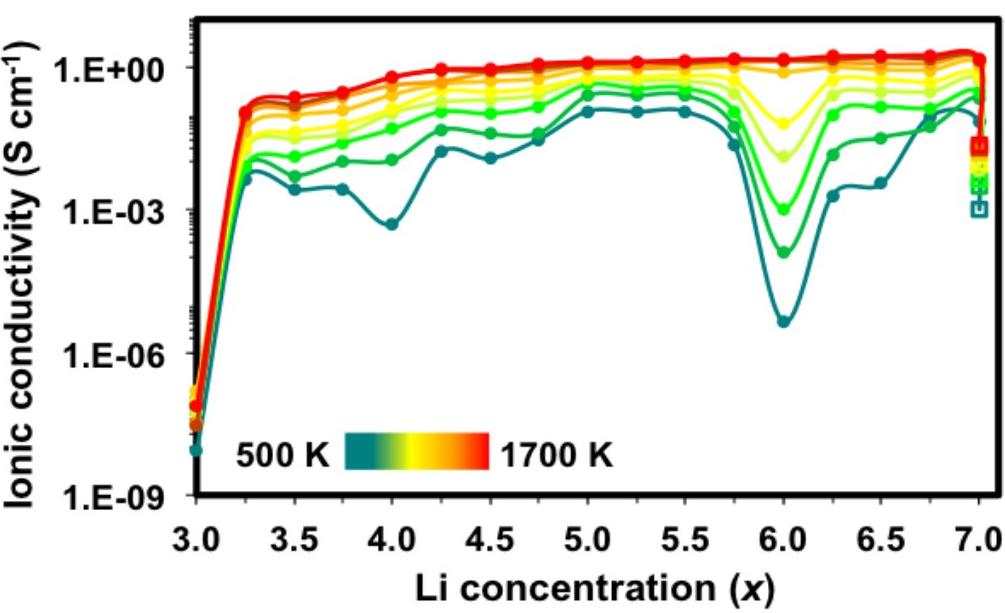
Fig. 3 - Ionic conductivity of LLZ garnet as a function of the concentration of Li+ ions, at various temperatures.
Similarly, for the high concentration x=7 the conductivity is low, because all octahedral sites are occupied, which makes the motion of Li ions difficult. When the concentration decreases the conductivity increases. This can be explained by the fact that the main carriers are the Li vacancies.
In summary, the ionic conductivity in LLZ garnet is controlled by the motion of Li+ ions in the low-concentration range x=3-5.5, and by the motion of lithium vacancies in the high-concentration range x=6.5-7. At a concentration x=6 the material has a particularly high-symmetry configuration, which explains that the conductivity drops dramatically around this concentration: neither lithium ions nor vacancies are able to move.
[1] B. Kozinsky et al., Phys. Rev. Lett. 116 (2016) 055901. doi: 10.1103/PhysRevLett.116.055901
Copyright © Pierre Hirel 2009-2024